From small to large
1. The larger biological molecules are made from smaller molecules. Polysaccharides are made from monosaccharides, proteins from amino acids, nucleic acids from nucleotides, lipids from fatty acids and glycerol.
2. Polysaccharides, proteins and nucleic acids are formed from repeating identical or similar subunits
called monomers, and are therefore polymers. These build up into large molecules called macromolecules.
3. The smaller units are joined together by condensation reactions. Condensation involves removal of water. The reverse process, adding water, is called hydrolysis and is used to break the large molecules back down into smaller molecules.
4. The linkages that join monosaccharides are called glycosidic bonds. Th e linkages that join amino acids are called peptide bonds.
Carbohydrates
5. Carbohydrates have the general formula Cx(H2O)y and comprise monosaccharides, disaccharides and polysaccharides.
6. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose) are very water-soluble and together are known as sugars.
7. Monosaccharides are the smallest carbohydrate units. Glucose is the most common. Th ey are important energy sources in cells and also important building blocks for larger molecules like polysaccharides.
8. Monosaccharides may have straight-chain or ring structures and may exist in diff erent isomeric forms such as α-glucose and β-glucose.
9. Benedict’s reagent can be used to test for reducing and non-reducing sugars. The test is semiquantitative.
10. Polysaccharides include starch, glycogen and cellulose.
11. Starch is an energy storage compound in plants. It is made up of two types of molecule, amylose and amylopectin, both made from α-glucose. Amylose is an unbranching molecule, whereas amylopectin has a branching structure. ‘Iodine solution’ can be used to test for starch.
12. Glycogen is an energy storage compound in animals, which is also made from α-glucose. Its structure is similar to that of amylopectin, but with more branching.
13. Cellulose is a polymer of β-glucose molecules. The molecules are grouped together by hydrogen bonding to form mechanically strong fi bres with high tensile strength that are found in plant cell walls.
Lipids
14. Lipids are a diverse group of chemicals, the most common of which are triglycerides (fats and oils).
15. Triglycerides are made by condensation between three fatty acid molecules and glycerol. Th ey are
hydrophobic and do not mix with water. They are energy storage compounds in animals, as well as having other functions such as insulation and buoyancy in marine mammals.
16. Phospholipids have a hydrophilic phosphate head and two hydrophobic fatty acid tails. Th is is
important in the formation of membranes.
17. The emulsion test can be used to test for lipids.
Proteins
18. Proteins are long chains of amino acids which fold into precise shapes. Th e sequence of amino acids in a protein, known as its primary structure, determines the way that it folds and hence determines its threedimensional shape and function.
19. Many proteins contain areas where the amino acid chain is twisted into an α-helix; this is an example of secondary structure. Th e structure forms as a result of hydrogen bonding between the amino acids. Another secondary structure formed by hydrogen bonding is the β-pleated sheet.
20. Further folding of proteins produces the tertiary structure. Often, a protein is made from more than
one polypeptide chain. Th e association between the diff erent chains is the quaternary structure of the
protein. Tertiary and quaternary structures are very precise and are held in place by hydrogen bonds,
disulfi de bonds (which are covalent), ionic bonds and hydrophobic interactions.
21. Proteins may be globular or fi brous. A molecule of a globular protein, for example haemoglobin, is roughly spherical. Most globular proteins are soluble and metabolically active. Haemoglobin contains a nonprotein (prosthetic) group, the haem group, which contains iron. Th is combines with oxygen. A molecule of a fi brous protein, for example collagen, is less folded and forms long strands. Fibrous proteins are insoluble. They often have a structural role. Collagen has high tensile strength and is the most common animal protein, being found in a wide range of tissues.
22. Biuret reagent can be used to test for proteins.
Water
23 Water is important within plants and animals, where it forms a large part of the mass of each cell. It is also an environment in which organisms can live.
24 As a result of extensive hydrogen bonding, water has unusual properties that are important for life: it is liquid at most temperatures on the Earth’s surface; its highest density occurs above its freezing point, so that ice fl oats and insulates water below from freezing air temperatures; it acts as a solvent for ions and polar molecules, and causes non-polar molecules to group together; it has a high surface tension, which aff ects the way it moves through narrow tubes and forms a surface on which some organisms can live. Water can also act as a reagent inside cells, as in hydrolysis reactions and in photosynthesis as a source of hydrogen.
Multiple - choice Test
1 The results of testing a solution for the presence of three biological molecules are shown in the table.
Which biological molecules are present in the solution?
A reducing sugar and protein
B reducing sugar and starch
C protein only
D starch only
2 The diagrams show the structure of four monosaccharides.
3 Which reaction involves the hydrolysis of glycosidic bonds?
A cellulose → glucose
B glucose → glycogen
C protein → amino acids
D triglyceride → fatty acids and glycerol
4 The diagram shows a tripeptide made from three glycine amino acids.
Which of the bonds numbered 1 to 8 represent peptide bonds?
A 1 and 7
B 2 and 8
C 3 and 6
D 4 and 5
5 The following bonds are among those found in proteins: disulfide, hydrogen, ionic and peptide bonds.
Which row shows the bonds involved in primary, secondary and tertiary protein structures?
6 Which of the following describes a molecule of haemoglobin?
A a central haem group enclosed by four coiled globin polypeptides
B a central globin group enclosed by four coiled haem polypeptides
C four coiled globin polypeptides, each with a central haem group
D four coiled haem polypeptides, each with a central globin group
7 Which statement about the properties of water is not correct?
A Evaporation of water is an effective means of cooling an organism.
B Large volumes of water are slow to change temperature as the environmental temperature changes.
C The solid form of water, ice, is more dense than the liquid form.
D Water is an excellent solvent for ions and polar molecules.
8 Amylopectin is formed from amylose by a plant cell detaching short lengths of an amylose chain and reattaching them as branches.
Which bonds are broken and which are formed when amylose is converted into amylopectin?
9 The graph shows the variation in melting point of triglycerides with different numbers of carbon atoms in their fatty acid chains.
What explains these results?
A Triglycerides with longer fatty acid chains have stronger intermolecular forces and so have a lower melting point.
B Triglycerides with longer fatty acid chains have weaker intermolecular forces and so have a higher melting point.
C Triglycerides with shorter fatty acid chains have stronger intermolecular forces and so have a higher melting point.
D Triglycerides with shorter fatty acid chains have weaker intermolecular forces and so have a lower melting point.
10 In spider silk, the polypeptide chains have the amino acid sequence Gly-Ala-Gly-Ala repeated many times, and the chains pack together as shown in the diagram. The diagram shows the R groups of the two amino acids.
Which of the following describes this structure?
A α-helix held together by hydrogen bonds
B α-helix held together by ionic bonds
C β-sheet held together by hydrogen bonds
D β-sheet held together by ionic bonds
Answer to multiple-choice test
1 A
2 C
3 A
4 C
5 B
6 C
7 C
8 B
9 D
10 C
End- of - chapter questions
1 Which term describes both collagen and haemoglobin?
A enzymes
B fibrousproteins
C globularproteins
D macromolecules
2 Whattype of chemical reaction is involved in the formation of disulfide bonds?
A condensation
B hydrolysis
C oxidation
D reduction
3 Which diagram best represents the arrangement of water molecules around sodium (Na") and chloride (CI-) ions in solution?
6 Statethree characteristics of monosaccharides.
7 The diagram shows a disaccharide called lactose. The carbon atoms are numbered. You are not expected to have seenthis structure before. Lactose is a reducing sugar found in milk. It is made from a reaction between the two monosaccharides glucose and galactose.
a Suggest two functions that lactose could have. [2]
b What is the name given to the reaction referred to above that results in the formation of lactose? [1]
c Identity the bond labelled X in the diagram. [1]
d Draw diagrams to show the structures of separate molecules of glucose and galactose. [2]
e Using the information in the diagram, is the alpha or beta form of glucose used to make lactose?
Explain your answer. [2]
f Like lactose, sucrose is a disaccharide. If you were given a solution of lactose and a solution of sucrose, state briefly how you could distinguish between them. [2]
8 a The diagram below shows the structures of three amino acids.
i Draw a diagram to show the structure of the tripeptide with the following sequence:
alanine-glycine-serine. [3]
ii What is the name given to the sequence of amino acids in a protein? [1]
iii What substance, apart from the tripeptide, would be formed when the three amino acids combine? [1]
iv Draw a ring around an atom or group of atoms making up an R group that could hydrogen bond with a neighbouring R group. [1]
v Draw a ring around and label the peptide bond(s) you have drawn in the diagram. [1]
vi Draw a ring around a group of atoms which could hydrogen bond with a -C=O group in an alpha helix. Label this group A. [1]
b State three features that a-helices and beta sheets have in common. [3]
c A protein can be described as a polymer. State the meaning of the term polymer. [2]
d X and Y represent two different amino acids.
i Write down the sequences of all the possible tripeptides that could be made with just these two amino acids.[1]
ii From your answer to d i, what is the formula for calculating the number of different tripeptides that can be formed from two different amino acids?[1]
9 Copy the diagrams below.
Answer to end-of-chapter questions
1 D
2 C
3 B
4
5
6 dissolve easily in water; sweet; general formula (CH2O)n/contain the elements carbon, hydrogen
and oxygen/hydrogen and oxygen are present in ratio of 2 : 1;
Exam-style questions
7
a lactose could be a source of energy; it could be digested to, monosaccharides/glucose and galactose, which could then be used as building blocks for larger molecules; [2]
b condensation; [1]
c glycosidic bond; [1]
d
8 a
i
C of COOH joined to N of NH2 for both
peptide bonds;
peptide bonds shown as C=O joined to –NH (i.e.
water has been eliminated);
all three amino acids joined and in correct sequence; A even if errors in bonding [3]
ii primary structure; [1]
iii water; [1]
iv ring drawn around –OH or whole R group (–CH2OH) of serine; [1]
v rings drawn around two peptide bonds and bonds labelled appropriately; [1]
vi ring drawn around –NH group one side of a peptide bond and group labelled; [1]
b held in place by hydrogen bonding; secondary structures; all the –NH and –C=O groups of, peptide
bonds/polypeptide backbone, are involved; [3]
c molecule made from repeating subunits; subunits similar or identical to each other; giant molecule/macromolecule; [max. 2]
d
i XXX, XXY, XYY, XYX, YYY, YYX, YXX, YXY; [1]
ii 23 [1]
9
a A identified as lipid, B identified as phospholipid; [1]
b i junction between head and tail for all three tails is indicated on diagram; allow 1 mark if only one or two junctions indicated [2]
ii fatty acids; glycerol; [2]
c head of phospholipid is labelled phosphate; [1]
d i phospholipid/B; [1]
ii phosphate is, charged/polar/hydrophilic; [1]
e lipid: energy store/insulator/buoyancy/source of metabolic water/any other suitable example;
phospholipid: any reference to the importance of phospholipids in structure of membranes; [2]
[Total: 10]
10 a
1 mark for each correct row. No half marks. [5]
b 1 mark for structural feature, 1 mark for linking this feature to its function, e.g.
haemoglobin contains iron;
iron combines with oxygen; [2]
c molecule has more than one polypeptide chain; [1]
R molecule has four polypeptide chains
d carbon, hydrogen, oxygen, nitrogen, iron;
2 marks for all five correct, 1 mark for four correct, 0 marks for 3 or fewer correct [2]
[Total: 10]
1. The larger biological molecules are made from smaller molecules. Polysaccharides are made from monosaccharides, proteins from amino acids, nucleic acids from nucleotides, lipids from fatty acids and glycerol.
2. Polysaccharides, proteins and nucleic acids are formed from repeating identical or similar subunits
called monomers, and are therefore polymers. These build up into large molecules called macromolecules.
3. The smaller units are joined together by condensation reactions. Condensation involves removal of water. The reverse process, adding water, is called hydrolysis and is used to break the large molecules back down into smaller molecules.
4. The linkages that join monosaccharides are called glycosidic bonds. Th e linkages that join amino acids are called peptide bonds.
Carbohydrates
5. Carbohydrates have the general formula Cx(H2O)y and comprise monosaccharides, disaccharides and polysaccharides.
6. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose) are very water-soluble and together are known as sugars.
7. Monosaccharides are the smallest carbohydrate units. Glucose is the most common. Th ey are important energy sources in cells and also important building blocks for larger molecules like polysaccharides.
8. Monosaccharides may have straight-chain or ring structures and may exist in diff erent isomeric forms such as α-glucose and β-glucose.
9. Benedict’s reagent can be used to test for reducing and non-reducing sugars. The test is semiquantitative.
10. Polysaccharides include starch, glycogen and cellulose.
11. Starch is an energy storage compound in plants. It is made up of two types of molecule, amylose and amylopectin, both made from α-glucose. Amylose is an unbranching molecule, whereas amylopectin has a branching structure. ‘Iodine solution’ can be used to test for starch.
12. Glycogen is an energy storage compound in animals, which is also made from α-glucose. Its structure is similar to that of amylopectin, but with more branching.
13. Cellulose is a polymer of β-glucose molecules. The molecules are grouped together by hydrogen bonding to form mechanically strong fi bres with high tensile strength that are found in plant cell walls.
Lipids
14. Lipids are a diverse group of chemicals, the most common of which are triglycerides (fats and oils).
15. Triglycerides are made by condensation between three fatty acid molecules and glycerol. Th ey are
hydrophobic and do not mix with water. They are energy storage compounds in animals, as well as having other functions such as insulation and buoyancy in marine mammals.
16. Phospholipids have a hydrophilic phosphate head and two hydrophobic fatty acid tails. Th is is
important in the formation of membranes.
17. The emulsion test can be used to test for lipids.
Proteins
18. Proteins are long chains of amino acids which fold into precise shapes. Th e sequence of amino acids in a protein, known as its primary structure, determines the way that it folds and hence determines its threedimensional shape and function.
19. Many proteins contain areas where the amino acid chain is twisted into an α-helix; this is an example of secondary structure. Th e structure forms as a result of hydrogen bonding between the amino acids. Another secondary structure formed by hydrogen bonding is the β-pleated sheet.
20. Further folding of proteins produces the tertiary structure. Often, a protein is made from more than
one polypeptide chain. Th e association between the diff erent chains is the quaternary structure of the
protein. Tertiary and quaternary structures are very precise and are held in place by hydrogen bonds,
disulfi de bonds (which are covalent), ionic bonds and hydrophobic interactions.
21. Proteins may be globular or fi brous. A molecule of a globular protein, for example haemoglobin, is roughly spherical. Most globular proteins are soluble and metabolically active. Haemoglobin contains a nonprotein (prosthetic) group, the haem group, which contains iron. Th is combines with oxygen. A molecule of a fi brous protein, for example collagen, is less folded and forms long strands. Fibrous proteins are insoluble. They often have a structural role. Collagen has high tensile strength and is the most common animal protein, being found in a wide range of tissues.
22. Biuret reagent can be used to test for proteins.
Water
23 Water is important within plants and animals, where it forms a large part of the mass of each cell. It is also an environment in which organisms can live.
24 As a result of extensive hydrogen bonding, water has unusual properties that are important for life: it is liquid at most temperatures on the Earth’s surface; its highest density occurs above its freezing point, so that ice fl oats and insulates water below from freezing air temperatures; it acts as a solvent for ions and polar molecules, and causes non-polar molecules to group together; it has a high surface tension, which aff ects the way it moves through narrow tubes and forms a surface on which some organisms can live. Water can also act as a reagent inside cells, as in hydrolysis reactions and in photosynthesis as a source of hydrogen.
Multiple - choice Test
1 The results of testing a solution for the presence of three biological molecules are shown in the table.
A reducing sugar and protein
B reducing sugar and starch
C protein only
D starch only
2 The diagrams show the structure of four monosaccharides.
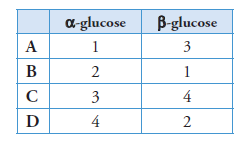
Which row in the table below identifies α-glucose and β-glucose?
3 Which reaction involves the hydrolysis of glycosidic bonds?
A cellulose → glucose
B glucose → glycogen
C protein → amino acids
D triglyceride → fatty acids and glycerol
4 The diagram shows a tripeptide made from three glycine amino acids.
Which of the bonds numbered 1 to 8 represent peptide bonds?
A 1 and 7
B 2 and 8
C 3 and 6
D 4 and 5
5 The following bonds are among those found in proteins: disulfide, hydrogen, ionic and peptide bonds.
Which row shows the bonds involved in primary, secondary and tertiary protein structures?
6 Which of the following describes a molecule of haemoglobin?
A a central haem group enclosed by four coiled globin polypeptides
B a central globin group enclosed by four coiled haem polypeptides
C four coiled globin polypeptides, each with a central haem group
D four coiled haem polypeptides, each with a central globin group
7 Which statement about the properties of water is not correct?
A Evaporation of water is an effective means of cooling an organism.
B Large volumes of water are slow to change temperature as the environmental temperature changes.
C The solid form of water, ice, is more dense than the liquid form.
D Water is an excellent solvent for ions and polar molecules.
8 Amylopectin is formed from amylose by a plant cell detaching short lengths of an amylose chain and reattaching them as branches.
Which bonds are broken and which are formed when amylose is converted into amylopectin?
9 The graph shows the variation in melting point of triglycerides with different numbers of carbon atoms in their fatty acid chains.
A Triglycerides with longer fatty acid chains have stronger intermolecular forces and so have a lower melting point.
B Triglycerides with longer fatty acid chains have weaker intermolecular forces and so have a higher melting point.
C Triglycerides with shorter fatty acid chains have stronger intermolecular forces and so have a higher melting point.
D Triglycerides with shorter fatty acid chains have weaker intermolecular forces and so have a lower melting point.
10 In spider silk, the polypeptide chains have the amino acid sequence Gly-Ala-Gly-Ala repeated many times, and the chains pack together as shown in the diagram. The diagram shows the R groups of the two amino acids.
Which of the following describes this structure?
A α-helix held together by hydrogen bonds
B α-helix held together by ionic bonds
C β-sheet held together by hydrogen bonds
D β-sheet held together by ionic bonds
Answer to multiple-choice test
1 A
2 C
3 A
4 C
5 B
6 C
7 C
8 B
9 D
10 C
End- of - chapter questions
1 Which term describes both collagen and haemoglobin?
A enzymes
B fibrousproteins
C globularproteins
D macromolecules
2 Whattype of chemical reaction is involved in the formation of disulfide bonds?
A condensation
B hydrolysis
C oxidation
D reduction
3 Which diagram best represents the arrangement of water molecules around sodium (Na") and chloride (CI-) ions in solution?
4 Copy and complete the following table. Place a tick or a cross in each box as appropriate.
5 Copy and complete the table below which summarises some of the functional categories into which proteins can be placed.
7 The diagram shows a disaccharide called lactose. The carbon atoms are numbered. You are not expected to have seenthis structure before. Lactose is a reducing sugar found in milk. It is made from a reaction between the two monosaccharides glucose and galactose.
a Suggest two functions that lactose could have. [2]
b What is the name given to the reaction referred to above that results in the formation of lactose? [1]
c Identity the bond labelled X in the diagram. [1]
d Draw diagrams to show the structures of separate molecules of glucose and galactose. [2]
e Using the information in the diagram, is the alpha or beta form of glucose used to make lactose?
Explain your answer. [2]
f Like lactose, sucrose is a disaccharide. If you were given a solution of lactose and a solution of sucrose, state briefly how you could distinguish between them. [2]
[Total: 10]
8 a The diagram below shows the structures of three amino acids.
i Draw a diagram to show the structure of the tripeptide with the following sequence:
alanine-glycine-serine. [3]
ii What is the name given to the sequence of amino acids in a protein? [1]
iii What substance, apart from the tripeptide, would be formed when the three amino acids combine? [1]
iv Draw a ring around an atom or group of atoms making up an R group that could hydrogen bond with a neighbouring R group. [1]
v Draw a ring around and label the peptide bond(s) you have drawn in the diagram. [1]
vi Draw a ring around a group of atoms which could hydrogen bond with a -C=O group in an alpha helix. Label this group A. [1]
b State three features that a-helices and beta sheets have in common. [3]
c A protein can be described as a polymer. State the meaning of the term polymer. [2]
d X and Y represent two different amino acids.
i Write down the sequences of all the possible tripeptides that could be made with just these two amino acids.[1]
ii From your answer to d i, what is the formula for calculating the number of different tripeptides that can be formed from two different amino acids?[1]
[Total: 15]
9 Copy the diagrams below.
a Identify with labels which one represents a lipid and which a phospholipid. [1]
b i For molecule A, indicate on the diagram where hydrolysis would take place if the molecule was digested. [2]
ii Name the products of digestion. [2]
c Each molecule has a head with tails attached. For molecule B, label the head to identify its chemical nature. [1]
d i Which of the two molecules is water-soluble?[1]
ii Explain your answer to d i.[1]
e State one function of each molecule. [2]
[Total: 10]
10 a Copy the following table to summarise some differences between collagen and haemoglobin.
Use the following to guide you.
Row l: State whether globular or fibrous
Row 2: State whether entirely helical or partly helical
Row 3: State the type of helix
Row 4: State whether a prosthetic group is present or absent
Row 5: State whether soluble in water or insoluble in water
Collagen
|
Haemoglobin
|
[5]
b State one way in which the structure of haemoglobin is related to its function. [2]
c Haemoglobin possesses a quaternary structure. What does this mean?[1]
d Name the five elements found in haemoglobin. [2]
[Total:10]
Answer to end-of-chapter questions
1 D
2 C
3 B
4
5
6 dissolve easily in water; sweet; general formula (CH2O)n/contain the elements carbon, hydrogen
and oxygen/hydrogen and oxygen are present in ratio of 2 : 1;
Exam-style questions
7
a lactose could be a source of energy; it could be digested to, monosaccharides/glucose and galactose, which could then be used as building blocks for larger molecules; [2]
b condensation; [1]
c glycosidic bond; [1]
d
glucose correctly drawn;
galactose correctly drawn; [2]
Carbon atoms need not be numbered. Note that galactose will probably be drawn ‘upside down’
as in the disaccharide – the conventional way of drawing it is also shown in the diagram above. The
form used to make the disaccharide is the beta form of galactose, but students will not need to know this, other than for interest.
e alpha glucose/α-glucose; the –OH group on carbon atom 1 is below the ring; [2]
f carry out a Benedict’s test on both solutions; lactose would give a brick-red/brown precipitate,
sucrose would not; accept positive result for lactose, negative result for sucrose [2]
[Total: 10]
i
C of COOH joined to N of NH2 for both
peptide bonds;
peptide bonds shown as C=O joined to –NH (i.e.
water has been eliminated);
all three amino acids joined and in correct sequence; A even if errors in bonding [3]
ii primary structure; [1]
iii water; [1]
iv ring drawn around –OH or whole R group (–CH2OH) of serine; [1]
v rings drawn around two peptide bonds and bonds labelled appropriately; [1]
vi ring drawn around –NH group one side of a peptide bond and group labelled; [1]
b held in place by hydrogen bonding; secondary structures; all the –NH and –C=O groups of, peptide
bonds/polypeptide backbone, are involved; [3]
c molecule made from repeating subunits; subunits similar or identical to each other; giant molecule/macromolecule; [max. 2]
d
i XXX, XXY, XYY, XYX, YYY, YYX, YXX, YXY; [1]
ii 23 [1]
9
a A identified as lipid, B identified as phospholipid; [1]
b i junction between head and tail for all three tails is indicated on diagram; allow 1 mark if only one or two junctions indicated [2]
ii fatty acids; glycerol; [2]
c head of phospholipid is labelled phosphate; [1]
d i phospholipid/B; [1]
ii phosphate is, charged/polar/hydrophilic; [1]
e lipid: energy store/insulator/buoyancy/source of metabolic water/any other suitable example;
phospholipid: any reference to the importance of phospholipids in structure of membranes; [2]
[Total: 10]
10 a
1 mark for each correct row. No half marks. [5]
b 1 mark for structural feature, 1 mark for linking this feature to its function, e.g.
haemoglobin contains iron;
iron combines with oxygen; [2]
c molecule has more than one polypeptide chain; [1]
R molecule has four polypeptide chains
d carbon, hydrogen, oxygen, nitrogen, iron;
2 marks for all five correct, 1 mark for four correct, 0 marks for 3 or fewer correct [2]
[Total: 10]





















there's a mistake
ReplyDeleteno: 7 in mcqs the answer cannot be C. as ice is actually less dense than water (liquid state)... that is why ice floats on water
ReplyDeletethnxx
This comment has been removed by a blog administrator.
DeleteNo it is C cuz the question asks which statement about water is not correct...
DeleteThank both of you. And as feyez al ahami have explained, the answer must be C. Sorry for anny inconvenient.
DeleteThan you. Very helpfull
ReplyDeletewasnt helpful :)
ReplyDeletewow thank you so much! This helps in my revision a lot <3
ReplyDeleteThanks
ReplyDeleteThanks this is very helpful
ReplyDelete