These factors are:
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- Inhibitor concentration
When an enzyme solution is added to a solution of its substrate, the molecules collide.
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- Inhibitor concentration
When an enzyme solution is added to a solution of its substrate, the molecules collide.
With time, the quantity of substrate ↓(changed into product) --> frequency of collisions ↓--> rate of reaction gradually ↓. The reaction rate is fastest at the start of the reaction (substrate concentration is greatest). --> When comparing reaction rates of an enzyme in different circumstances, we should
measure the initial rate of reaction.
1. Temperature
- As to ↑, kinetic energy of reacting molecules ↑--> ↑ successful collision --> ↑ rate of reaction.
- At optimal to enzyme's activity is maximal --> rate is maximal.
- Above this temperature, H bonds holding enzyme molecule in shape begin to break --> change tertiary structure of the enzyme (denaturation) --> active site is deformed ---> ↓binding of substrate with enzyme --> ↓ rate of reaction.
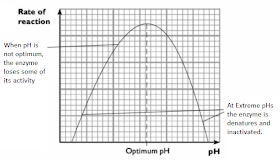
2. pH
- Most enzyme molecules only maintain their correct tertiary structure (exhibit maximum activity) within a very narrow pH range.
- Optimum pH - is the pH at which an enzyme has maximum activity. Biological buffers help maintain the optimum pH for an enzyme.
- Changes in pH can make and break intra- and intermolecular bonds, changing the shape of the enzyme and, therefore, its effectiveness.
- Most enzymes have an optimum pH that falls within the physiological range of 7.0-7.5.
- Notable exceptions are the digestive enzymes pepsin and trysin:
pepsin (active in the stomach) - optimum pH of 1.5
trypsin (active in the small intestine) - optimum pH of 8.0.
3. Enzyme concentration
When there are more substrate than enzyme:
When there are more substrate than enzyme:
- limiting factor is Enzyme concentration
- ↑ concentration of enzyme --> ↑ collisions between enzyme and substrate -->↑ rate of the reaction (directly proportional to the enzyme concentration )
Increasing the enzyme concentration beyond a certain point does not change the rate of reaction BECAUSE when there are less substrate than enzyme:
- limiting factor is Substrate concentration
- ↑ concentration of enzyme does NOT ↑ rate of reaction.
Limiting
factor:
- Factor that directly affects the rate of reaction at which a process occurs if its quantity is changed.
- Its value has to be ↑ in order to ↑ the rate.
4. Substrate concentration
When there are more enzyme than substrate:
- limiting factor is Substrate concentration
- ↑ concentration of substrate --> ↑ collisions between enzyme and substrate -->↑ rate of the reaction
Increasing the substrate concentration beyond a certain point does not change the rate of reaction BECAUSE when there are less enzyme than substrate:
- limiting factor is Enzyme concentration
- ↑ concentration of substrate does NOT ↑ rate of reaction.
5. Inhibitor concentration
Inhibitor = a substance that slows down the rate at which an enzyme works.
Competitive inhibitors
Investigating factors affecting the rate of enzyme-catalysed reactions
2. pH
3. Enzyme concentration
4. Substrate concentration
Competitive inhibitors
- Have similar shape to the enzyme's normal substrate.
- Can fit into the enzyme's active site, preventing the substrate from binding.
- The greater the proportion inhibitor : substrate, the more inhibitor molecules (not substrate molecules) will bump into an active site.
- Relative concentrations of the inhibitor and the substrate will affect the degree to which a competitive inhibitor slows down a reaction.
- Have different shape than the substrate.
- Do not bind to the active site.
- Bind to a different part of the enzyme --> changes the enzyme's shape (including the active site) --> substrate can not bind with enzyme.
- Relative concentrations of the inhibitor and the substrate does not affect the degree to which a non-competitive inhibitor slows down a reaction (if you add more substrate, it still won't be able to bind).
1. Temperature
The effect of to on enzyme activity
You can use almost any enzyme reaction for this, such as the action of catalase on H2O2 in the #17 post. You could use the same method of collecting the gas that is described there, but here is another possible method:
|
2. pH
The effect of pH on enzyme activity
|
3. Enzyme concentration
The effect of enzyme concentration on rate of reaction
You could use the following method to investigate the effect of enzyme concentration on the rate at which the enzyme catalase converts its substrate H2O2 to H2O and O2 .
|
4. Substrate concentration
The effect of substrate concentration on enzyme activity
You can do
this in the same way as described for investigating the effect of enzyme
concentration, but this time keep the concentration of catalase the same and
vary the concentration of hydrogen peroxide.
|
Enzimes and reactions
Following the course of an enzyme-catalysed reaction
Syllabus 2015
(d) [PA] investigate
and explain the effects of temperature, pH, enzyme concentration and
substrate concentration on the rate of enzyme-catalysed reactions;
(e) explain the
effects of competitive and non-competitive inhibitors on the rate of enzyme
activity;
|
Syllabus 2016 - 2018
Factors that affect enzyme action
Investigating the effects of factors on enzyme activity gives opportunities for planning and carrying out experiments under controlled conditions. a) investigate and explain the effects of the following factors on the rate of enzyme-catalysed reactions: • temperature • pH (using buffer solutions) • enzyme concentration • substrate concentration • inhibitor concentration c) explain the effects of reversible inhibitors, both competitive and non-competitive, on the rate of enzyme activity |










No comments:
Post a Comment